190K in Michigan live with Alzheimer’s. FDA taps brake on new drug.
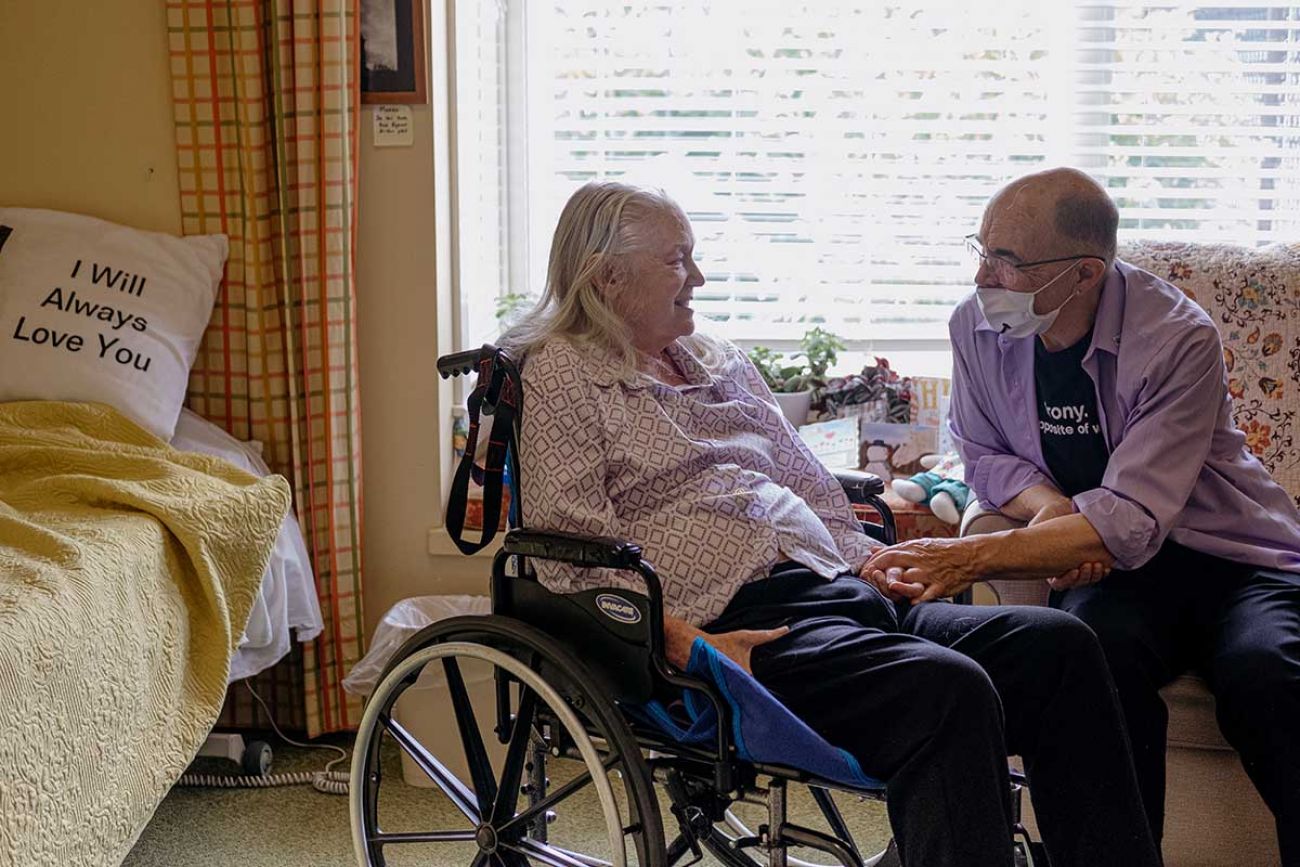
A controversial new drug to treat Alzheimer’s will now be limited only to those in the earliest states of the disease.
The U.S. Food and Drug Administration Thursday changed its June decision that would have made Aduhelm available to nearly anyone with Alzheimer’s — a wide-open invitation to some 6.2 million Americans living with the deadly dementia, according to estimates by the Alzheimer’s Association.
“Treatment … should be initiated in patients with mild cognitive impairment or mild dementia stage of disease, the population in which treatment was initiated in clinical trials,” according to new prescribing recommendations released Thursday.
Related:
- Hope or hype? Costly new Alzheimer’s drug creates controversy
- Overstressed and unpaid, 1.3M in Michigan care for relatives amid aging crisis
- As Michigan ages, one woman has made it her mission to train family caregivers
The FDA approved Aduhelm in June on an accelerated basis, based on preliminary results from three clinical trials — all involving people in early stages of the disease.
Limiting the drug makes sense, said Jim Mangi, of Saline, an ambassador for the Alzheimer’s Association of Michigan. His wife, a former scientist, lives in a memory care village now, 14 years after the family first noticed her symptoms. He visits her every day.
Mangi, himself a former scientist, has been watching the drug’s development, too. The drug has been tested — and shown to be possibly effective — only among those in early stages of the disease, he noted.
It only makes sense to prescribe Aduhelm to those whom it may be effective.

“A bucket of water can help with a campfire, not so much against a wildfire,” Mangi said.
The decision Thursday also was lauded by the Alzheimer’s Association’s chief science officer.

“We appreciate the FDA’s thoughtful consideration and response to the Alzheimer’s Association and others in the community including physicians, researchers and patients to ensure this treatment is prescribed only to those who may see benefit,” said Maria Carrillo in a statement.
The prescribing limit also will ease conversations between doctors and their patients desperate for treatment, said Jennifer Lepard, president and CEO of the Michigan chapter of the Alzheimer’s Association.
“It clarifies for people who will benefit” from the drug,” she said. “It helps with that communication.”
The association had lobbied for the drug’s approval — a controversial decision by the FDA — as well as limits to the drug’s use.
The drug has been hailed as possibly the first to stop the progression of the disease, according to its manufacturer, Massachusetts-based Biogen. A handful of earlier drugs only temporarily treat memory loss and cognitive function.
The drug works by breaking down sticky protein fragments called beta-amyloids that have clumped together in the brain over time to form plaques — a hallmark of Alzheimer’s disease. Lodged between neurons, these plaques appear to disrupt cell-to-cell communication and ultimately lead to cell death.
Also known as aducanumab, Aduhelm reduces the plaques, according to positron emission tomography (PET) images in studies involving 3,482 patients.
Still unresolved is this: It’s not immediately clear whether breaking down the plaques will halt or reverse the disease. The scans provide only a surrogate endpoint — offering evidence that the drug might work.
The evidence was so thin that some doctors groups said the drug’s approval was premature, including the American Geriatrics Society, which called the trials “inconclusive” and the data “limited.”
Even the FDA noted “residual uncertainties regarding clinical benefit.”
The drug is also exorbitantly expensive — $56,000 for a one-year supply of the drug, and that doesn’t account for costs to administer the infusion each month or so, or for follow-up care.
Medicare, the federal health insurance for people 65 or older, likely will be the largest insurer of Aduhelm. Even by conservative estimates, the tab would run into billions of dollars if it were available to anyone with Alzheimer’s. Local insurers also will decide if they will cover the cost.
See what new members are saying about why they donated to Bridge Michigan:
- “In order for this information to be accurate and unbiased it must be underwritten by its readers, not by special interests.” - Larry S.
- “Not many other media sources report on the topics Bridge does.” - Susan B.
- “Your journalism is outstanding and rare these days.” - Mark S.
If you want to ensure the future of nonpartisan, nonprofit Michigan journalism, please become a member today. You, too, will be asked why you donated and maybe we'll feature your quote next time!





